Document Control Software
In today’s fast-paced business environment, efficient document control is paramount, especially for organizations operating in regulated industries. Manual or hybrid systems are prone to errors, causing delays and potentially jeopardizing product quality, leading to regulatory penalties or ISO Non-conformance. A digital solution offers automation and streamlines your processes, ensuring effortless compliance with the latest regulatory changes.
Introducing QISS QMS Document Control Software, designed to improve your document management.

Our Clients













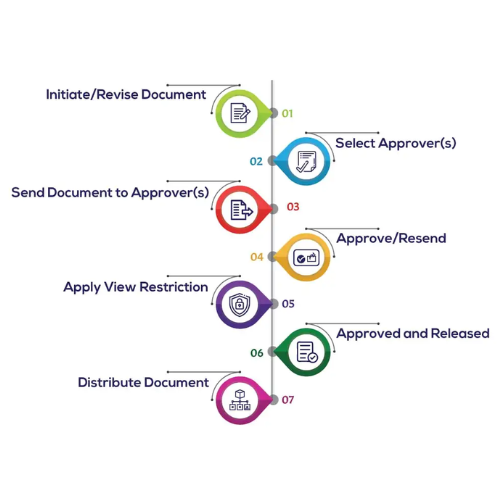
Solve Document Control Challenges with Our Software
Document control in quality management is challenging due to issues like version control, accessibility, regulatory compliance, and document security. Organizations also struggle with standardization, employee training, and managing outdated documents. Our software is designed to help you overcome these challenges by providing centralized, secure, and user-friendly tools to streamline document control processes and ensure compliance.
Benefits of Our Automated Document Control Software
Transitioning to a digital document control system benefits not only document control but also related processes within your quality management system (QMS). This interconnectivity streamlines interactions between different aspects of a QMS, simplifying training, corrective and preventive action (CAPA), supplier management, audits, and more. Here are key benefits of using document control software:
Maintain Regulatory Compliance
Our document control software automates all document types—SOPs, Policies, Manuals, Forms, and more—ensuring compliance by streamlining creation, approval, and tracking.
Increase Efficiency
Free your employees from chasing documents, boosting productivity and efficiency. Enjoy the cost-saving benefits of sustainability by going paperless.
Team Collaboration
Enable controlled access to the latest document versions for employees, suppliers, and contractors, regardless of location. Eliminate confusion arising from multiple communication channels.
Audit Readiness
Be audit ready all the time by maintaining all documents in a centralized software with time-stamped audit trails and 21 CFR Part 11 compliant electronic signatures.
Features of Document Control Software
Our document control software comes packed with powerful features designed to keep your operations organized, compliant, and efficient. Easily manage, track, and secure your documents with features like version control, access permissions, real-time updates, and audit trails—all in one centralized system.
Access Control
It provides secure access to documents, ensuring that only authorized personnel can view and edit sensitive information.
e-Signature
Ensure the authenticity and validity of electronic signatures, meeting regulatory requirements and streamlining approval processes.
Version control
Effectively manage document versions, track changes, and guarantee everyone works with the latest iteration, preventing costly errors.
Audit Trails
Track all document-related activities and history for audits and compliance purposes, simplifying the auditing process.
Approval system
Streamline the document review and approval process through automated workflows, electronic notifications and reminders.
Dashboard
Provide users with an overview of the number of documents pending approval, recent activity, and bottlenecks in the review process.
Exclusive Features: View & Edit Document
You can view and edit the document within our software. It allows you to both view and edit a document within the same platform. This means you can open the document to read its contents and simultaneously make changes, such as editing text, formatting, or adding new elements, all in real time. To know more, get in touch with us.

Affordable Software, Premium Performance
Powerful tools that fit your budget—no compromises, just results.
Document Control
Document Control for Manufacturing
Document control software is essential for manufacturing companies, especially those in heavily regulated industries. This software helps manage a wide array of documents, including standard operating procedures (SOPs), work instructions, engineering drawings, and quality manuals. By automating document control processes like review, approval, and distribution, manufacturers can reduce errors, improve efficiency, and speed up production cycles, all while ensuring compliance with industry-specific regulations such as ISO 9001 and FDA regulations. A robust document control system also provides a comprehensive audit trail, which is crucial for demonstrating compliance during audits and inspections.
Laboratory Document Control Software
Laboratories, especially in life sciences, handle many critical documents. Specialized laboratory document control software helps manage these documents efficiently while ensuring compliance with regulatory standards. Key features include version control, which tracks changes to documents like test methods and protocols; comprehensive audit trails that provide evidence of compliance; and secure access to protect sensitive data. Integration with Laboratory Information Management Systems (LIMS) streamlines data management and workflow automation.
Document Control for Small Businesses
Document control software is not just for large enterprises; it can be equally valuable for small businesses. Cloud-based document control solutions offer cost-effectiveness, scalability, and ease of use, making them ideal for smaller companies. These solutions eliminate the need for expensive hardware and IT infrastructure and can readily adapt to a growing business's needs. User-friendly interfaces make it easy for employees to adopt and utilize the system effectively.
Document Control in QMS
Document control is a foundational element of any effective quality management system (QMS). It ensures that all quality-related documents are controlled, accurate, and up-to-date, contributing to standardized processes, compliance with regulations and industry standards, and continuous improvement efforts. A well-maintained document control system supports audits by providing auditors with clear evidence of compliance with quality standards. This control is essential for demonstrating that an organization consistently follows procedures, adheres to quality guidelines, and actively seeks ways to enhance its processes.
ISO Documentation Software
ISO documentation software helps organizations meet the documentation requirements of various ISO standards, including ISO 9001 for quality management, ISO 14001 for environmental management, and ISO 27001 for information security management. The software provides a centralized repository for managing all ISO-related documents. Robust version control and audit readiness features are crucial for demonstrating continuous improvement and maintaining compliance with the chosen ISO standard.
About QIA

Years of Experience
Proven track record of helping businesses improve efficiency, compliance, and profitability.
ISO Trainer & Consultants
Our software is built with expert guidance from ISO trainers to simplify compliance.

Internal ISO Auditor
Our internal auditors helped us incorporate all the essential features to ensure a successful audit.

Purpose-Built Software
Our software is specifically designed to fit your unique needs and stay ahead of compliance challenges.
Testimonials
What Our Clients Say About Us
Reduce Stress and Streamline Your Document Control Process with QISS QMS
Here, you will find articles related to different aspects of a quality management and document control. This is a center for gaining knowledge.